STUDIES OF HYDROXYLAPATITE CRYSTAL MORPHOLOGY: A COMBINED INFRARED AND B3LYP STUDY
Сучасне матеріалознавство та товарознавство: теорія, практика, освіта :: АКТУАЛЬНІ ПИТАННЯ НАУКОВОГО ТА ПРАКТИЧНОГО МАТЕРІАЛОЗНАВСТВА
Страница 1 из 1
 STUDIES OF HYDROXYLAPATITE CRYSTAL MORPHOLOGY: A COMBINED INFRARED AND B3LYP STUDY
STUDIES OF HYDROXYLAPATITE CRYSTAL MORPHOLOGY: A COMBINED INFRARED AND B3LYP STUDY
Fabio Chiatti, Yuriy Sakhno, Marta Corno, Gianmario Martra,
Piero Ugliengo Dipartimento di Chimica & Centro di Eccellenza NIS, Università di Torino, Italy
STUDIES OF HYDROXYLAPATITE CRYSTAL MORPHOLOGY:
A COMBINED INFRARED AND B3LYP STUDY
ABSTRACT
In this work we have studied the adsorption process of CO on hydroxyapatite (HA), material of interest as biomimetic counterpart of
inorganic phase of bones and teeth [1] and as catalyst/catalyst support [2,3]. The peculiarities of the CO molecule (low reactivity but high selectivity towards adsorbing sites) make it one of the most common molecule to probe the surfaces features of inorganic materials. Theoretical models, simulated at a B3LYP level with the CRYSTAL code, FT-IR and high resolution transmission electron microscopy (HR-TEM) experiments have been adopted to study the adsorption processes of CO at HA surfaces.
Theoretically, we have considered four distinct slab models as HA termination: the stoichiometric HA(001) and HA(010)R (the R stands for “water-reacted”) surfaces and the non-stoichiometric HA(010)_Ca-rich and HA(010)_P-rich surfaces. The adsorption of CO at those surfaces has been studied by simulating the CO loading from 1 to 4 CO per unit cell (u.c.). After the geometry optimization, energetic and vibrational features have been evaluated. The enthalpy calculated at room temperature decreases with the increasing CO loading, from about 30 kJ∙mol-1 at lowest loading (1 CO/u.c.), towards a plateau at 20 kJ∙mol-1 for the highest coverage (4 CO/u.c.). The same decreasing trend, here interpreted by the opposite behaviours of electronic and dispersive contributions, has already been observed, but not completely understood, in previous microcalorimetric experiments [4]. By a careful comparison between IR experimental spectrum of CO adsorbed on HA nanoparticles mainly exposing (010) facets (by HR-TEM) [5] and the spectra computed at B3LYP level for the considered HA terminations, it turned out that only the CO bands deriving from the CO adsorbed on HA(010)_Ca-rich and the HA(010)_P-rich terminations are matching the experimental band (see Figure 1). This is a strong indication that the interplay between quantum mechanical calculations and IR spectra allows to clarify the crystalline habit of nanostructured biomaterials/catalyst which may be very relevant for the biological activity/reactivity.
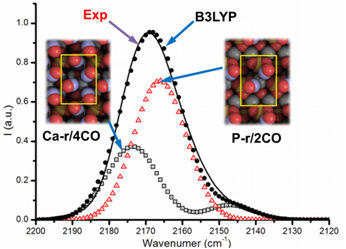 Figure 1. Comparison between experimental and theoretical IR spectra. The black solid line represents the experimental spectrum; the red-triangles and the black-squares curves represent the spectra of the 2CO/HA(010)_P-rich=P-r/2CO and the 4CO/HA(010)_Ca-rich=Ca-r/4CO models, respectively; the sum of these two spectra originates the black-dot spectrum, which represents the total B3LYP spectrum.
Figure 1. Comparison between experimental and theoretical IR spectra. The black solid line represents the experimental spectrum; the red-triangles and the black-squares curves represent the spectra of the 2CO/HA(010)_P-rich=P-r/2CO and the 4CO/HA(010)_Ca-rich=Ca-r/4CO models, respectively; the sum of these two spectra originates the black-dot spectrum, which represents the total B3LYP spectrum.
References: [1] Dorozhkin, S. Biology and Medicine Materials, 2009, 2, 1975-2045. [2] Xu, J.; White, T.; Li, P.; He, C.; Han, Y.; J. Am. Chem. Soc. , 2010, 132, 13172–13173. [3] Diallo-Garcia, S.; Laurencin, D.; Krafft, J. M.; Casale, S.; Smith, M. E.; Lauron-Pernot, H.; Costentin, G. J. Phys. Chem. C 2011, 115, 24317-24327.[4] Bolis, V.; Busco, C.; Martra, G.; Bertinetti, L.; Sakhno, Y.; Ugliengo, P.; Chiatti, F.; Corno, M.; Roveri, N. Phil. Trans. R. Soc. A 2012, 370, 1313. [5] Sakhno, Y.; Bertinetti, L.; Iafisco, M.; Tampieri, A.; Roveri, N.; Martra, G. J. Phys. Chem. C, 2010, 114, 16640-16648.
Piero Ugliengo Dipartimento di Chimica & Centro di Eccellenza NIS, Università di Torino, Italy
STUDIES OF HYDROXYLAPATITE CRYSTAL MORPHOLOGY:
A COMBINED INFRARED AND B3LYP STUDY
ABSTRACT
In this work we have studied the adsorption process of CO on hydroxyapatite (HA), material of interest as biomimetic counterpart of
inorganic phase of bones and teeth [1] and as catalyst/catalyst support [2,3]. The peculiarities of the CO molecule (low reactivity but high selectivity towards adsorbing sites) make it one of the most common molecule to probe the surfaces features of inorganic materials. Theoretical models, simulated at a B3LYP level with the CRYSTAL code, FT-IR and high resolution transmission electron microscopy (HR-TEM) experiments have been adopted to study the adsorption processes of CO at HA surfaces.
Theoretically, we have considered four distinct slab models as HA termination: the stoichiometric HA(001) and HA(010)R (the R stands for “water-reacted”) surfaces and the non-stoichiometric HA(010)_Ca-rich and HA(010)_P-rich surfaces. The adsorption of CO at those surfaces has been studied by simulating the CO loading from 1 to 4 CO per unit cell (u.c.). After the geometry optimization, energetic and vibrational features have been evaluated. The enthalpy calculated at room temperature decreases with the increasing CO loading, from about 30 kJ∙mol-1 at lowest loading (1 CO/u.c.), towards a plateau at 20 kJ∙mol-1 for the highest coverage (4 CO/u.c.). The same decreasing trend, here interpreted by the opposite behaviours of electronic and dispersive contributions, has already been observed, but not completely understood, in previous microcalorimetric experiments [4]. By a careful comparison between IR experimental spectrum of CO adsorbed on HA nanoparticles mainly exposing (010) facets (by HR-TEM) [5] and the spectra computed at B3LYP level for the considered HA terminations, it turned out that only the CO bands deriving from the CO adsorbed on HA(010)_Ca-rich and the HA(010)_P-rich terminations are matching the experimental band (see Figure 1). This is a strong indication that the interplay between quantum mechanical calculations and IR spectra allows to clarify the crystalline habit of nanostructured biomaterials/catalyst which may be very relevant for the biological activity/reactivity.

References: [1] Dorozhkin, S. Biology and Medicine Materials, 2009, 2, 1975-2045. [2] Xu, J.; White, T.; Li, P.; He, C.; Han, Y.; J. Am. Chem. Soc. , 2010, 132, 13172–13173. [3] Diallo-Garcia, S.; Laurencin, D.; Krafft, J. M.; Casale, S.; Smith, M. E.; Lauron-Pernot, H.; Costentin, G. J. Phys. Chem. C 2011, 115, 24317-24327.[4] Bolis, V.; Busco, C.; Martra, G.; Bertinetti, L.; Sakhno, Y.; Ugliengo, P.; Chiatti, F.; Corno, M.; Roveri, N. Phil. Trans. R. Soc. A 2012, 370, 1313. [5] Sakhno, Y.; Bertinetti, L.; Iafisco, M.; Tampieri, A.; Roveri, N.; Martra, G. J. Phys. Chem. C, 2010, 114, 16640-16648.
Сучасне матеріалознавство та товарознавство: теорія, практика, освіта :: АКТУАЛЬНІ ПИТАННЯ НАУКОВОГО ТА ПРАКТИЧНОГО МАТЕРІАЛОЗНАВСТВА
Страница 1 из 1
Права доступа к этому форуму:
Вы не можете отвечать на сообщения|
|
|
